On October 31, 2019, the Centers for Medicare & Medicaid Services (CMS) released its 2020 End Stage Renal Disease/Durable Medical Equipment Payment final rule. In addition to changes to payments under the end-stage renal disease (ESRD) and durable medical equipment (DME) programs, the rule includes changes to payment for renal dialysis, the ESRD quality incentive program, DME competitive bidding, and DME orders and prior authorization.
CMS anticipates that this rule will increase overall payments to ESRD facilities by 1.7 percent, for an increase of $210 million in 2020. CMS cannot estimate the impact of its changes to the DME rate setting and ordering methodologies.
Provisions of the final rule will go into effect on January 1, 2020.
ESRD Base Rate Raised by $4
Payment for ESRD services is made via a bundled prospective payment system adjusted for patient complexity. The base rate, which serves as the basis upon which other payments can be made, is adjusted annually to account for inflation. For 2020, CMS will increase the ESRD base rate from $235.27 to $239.33, reflecting a 1.7 percent market adjustment and a 1.00418 percent budget-neutrality factor. The base payment for acute kidney injury (AKI) would be adjusted to the same rate.
Outlier Thresholds Lowered for 2020, CMS Hopes to Double Outlier Cases
Because CMS did not reach its 2018 goal of 1 percent of ESRD payments qualifying for outlier payment, the threshold for outlier payments will lower in 2020, meaning more beneficiaries should qualify. The fixed-dollar loss (FDL) outlier threshold for pediatric patients will decrease from $57.14 to $41.04 and the Medicare Allowable Payment (MAP) will decrease from $38.51 to $35.78. For adult patients, the FDL will decrease from $65.11 to $48.33 and the MAP will decrease from $38.51 to $35.78. The goal is to increase the number of Medicare beneficiaries qualifying as outliers from 0.5% (current rate) to 1%.
New Add-on Payment for Innovative Dialysis Equipment and Supplies Approved January 1, 2020 or Later
CMS is finalizing a new add-on payment for dialysis equipment and supplies that are novel and demonstrate substantial clinical improvement (as with the new-technology add-on payments under the inpatient fee schedule). To qualify, equipment and supplies must have been designated by CMS as a renal dialysis service; be granted FDA marketing authorization on or after January 1, 2020; and are commercially available by January 1 of the year in which the payment adjustment would take effect; and meet the “substantial clinical improvement criteria” outlined in the inpatient new technology add-on payment system. This payment adjustment would be equivalent to 65 percent of the fee established by the DME Medicare Administrative Contractors (MACs), and would be paid for two years, after which time it would instead qualify as an outlier service.
Changes for Transitional Drug Add-on Payments (TDAPA), Lower Payment for Calcimimetics in 2020
CMS currently makes add-on payments for new renal dialysis drugs and biological products which may not yet be fully integrated into the pricing for an ESRD service category. For 2020, CMS will exclude drugs submitted under an abbreviated drug application (ANDA) or under various types of new drug applications that may indicate a similarity to existing drugs.
For calcimimetics, CMS will continue payment for a third year, but will reduce the add-on payment from the average sales price (ASP) plus six percent (106% of ASP) to ASP alone (100% of ASP). This will match the new rate, effective 2020, for all new TDAPA drugs, of 100 percent of ASP. CMS will also eliminate the erythropoiesis-stimulating agent (ESA) monitoring policy (EMP), as these agents are now incorporated into the per treatment payment amount.
Standards for Gap-Filling New DME Pricing Clarified
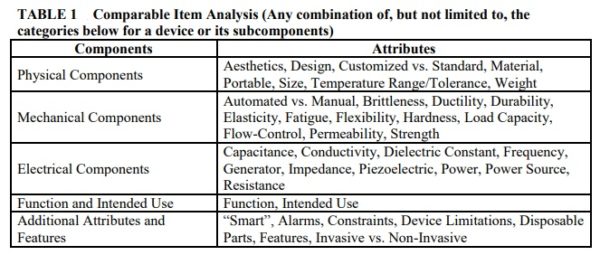
CMS finalizes the criteria by which it may determine that newly Medicare covered items are comparable to items used in the gap-filling pricing process. CMS engages in a process known as “gap-filling” in order set prices for new durable medical equipment covered under Medicare. This process sets prices in the Medicare fee schedule for these items based on outside data sources on similar and identical technology. CMS has received criticism from industry for the lack of transparency in the gap-filling process in the past, specifically on how it determines which technology is similar and what pricing data is used.
CMS finalizes five main categories by which it may determine that new DMEPOS Items can be compared to older DMEPOS items. New products do not need to be comparable within each category and there is no prioritization of the categories.
CMS finalizes that it will use commercial price lists and supplier invoices to determine the fee schedule amount for a new item if a comparable device does not exist. CMS had proposed using technology assessment as another potential source of information but did not finalize it based on concerns from stakeholders.
In addition, CMS finalizes a one-time adjustment to gap-filled fee schedule items if the supplier or commercial prices for a new item decrease by less than 15 percent within 5 years of establishing the initial fee schedule amount. In such cases, gap-filling will be conducted a second time to reduce the fee schedule amount by up to 14.99 percent. CMS finalizes the one-time adjustment due to the concerned that, for new categories of DME where there are few or only one manufacturers or suppliers, initial prices may not yet reflect a competitive marketplace. There will be no price adjustments made in cases where prices increase.
Master List of DME Subject to Heightened Scrutiny Finalized
CMS streamlines overlapping prior regulatory DMEPOS payment requirements for DME suppliers in order to curb suspected erroneous claim submission by combining them into a single “Master List.” The requirement lists created by former rules include:
- Face-to-face and prescription requirements for power mobility devices;
- Separate face-to-face and prescription requirements for other items of DME;
- Additional subregulatory requirements for other items of DME; and
- Prior authorization requirements.
The “Master List of DMEPOS Items Potentially Subject to Face-to-Face Encounter and Written Order Prior to Delivery and/or Prior Authorization Requirements” will serve as a library of items from which an item may be selected to apply these requirements. The Master List has 413 items and will be updated annually.
Additionally, CMS finalizes that Medicare Contractors must consider the “totality of the medical record” standard when reviewing written orders for compliance prior to delivery, rather than the current, stricter requirements.
CMS Continues Change of Ownership Process in the Competitive Bidding Program
CMS removes the requirement for contract suppliers under the DMEPOS Competitive Bidding Program to notify the Agency 60 days in advance of a change of ownership (CHOW). CMS will replace that requirement with a policy to notify the Agency no later than 10 days after the effective date of the change.


